Experiment of The Month
The Chick Waterer, the Soda Bottle and the Ping Pong Ball
Older readers may recognize the device below as a water supply for free range chickens. The chicken drinks from the metal dish, and when the water level in the dish gets low, a bubble rises into the water bottle and more water leaks into the dish. The effect is the same as an office bottled water dispenser, but more visible.


On the right, the waterer is disassembled, showing the cap that screws onto the jar. The holes in the cap allow water flow until the pressure on both sides of the hole is the same. Neglecting surface tension, this happens when two conditions are satisfied:
- The water level in the metal dish is high enough to cover the holes.
- The air pressure inside the jar is low enough to make the pressure at the bottom of the water column in the jar equal to atmospheric pressure (plus a slight additional pressure due the water level in the dish).
Dr. Cooney saw a demonstration by Courtney Willis et al. at the 2005 summer American Association of Physics Teachers meeting that turns this effect into a measurement of atmospheric pressure. Willis et al. used a soda bottle (Sobe 20 oz. glass bottles work best) and a ping pong ball. Dr. Cooney was inspired to introduce the experiment into our introductory physics laboratory. The photos below were taken during one of Dr. Cooney's laboratory classes.
Below on the left, a ping pong ball provides the seal and atmospheric pressure holds the ball against the glass so that no water leaks out. Below on the right is the tricky part.


To start, the bottle is filled about 2/3 full. The ball is held (fingers on side) against the glass and the bottle tipped up. The ball is held loosely against the glass so that water is allowed to leak out. This dripping water is collected in a beaker. Eventually the air pressure inside the bottle falls to a low enough value that the pressure on both sides of the ball is equal, and the hand can be removed.
For the determination of atmospheric pressure, refer to the diagram below.
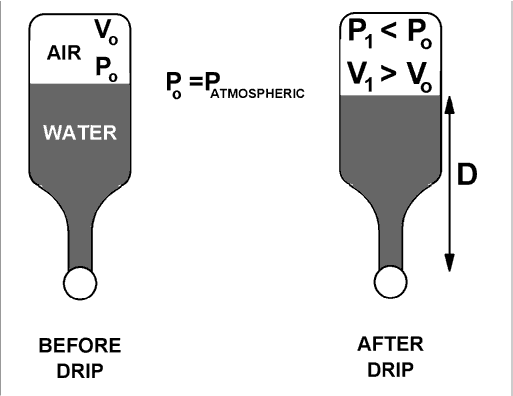
The initial volume, Vo, is measured as follows: Fill the bottle to the brim, press the ball down to squeeze a little water out, and then pour about 1/3 of the remaining water into a beaker. Determine the volume of that poured water either with a graduated cylinder or by weighing. That volume is Vo.
Now put the ping pong ball back against the opening and do the tricky part of letting water drip out past the ball into a beaker. This small "drip volume" of water that collects in the beaker is the difference between V1 and Vo. Measure the "drip volume" and add it to Vo to find a value for V1 . (The final volume V1 is not as much larger as the figure suggests.)
Since the pressure is the same on both sides of the ball,
P1 + rg D = PATMOSPHERIC = Po
where r is the density of water and g is the gravitational field strength. (Remember that Po was also atmospheric pressure.) Since air is nearly an ideal gas,
P1 V1 =PoVo is a good approximation.
Setting P1 = Po(Vo/V1 ) and solving for the atmospheric pressure, Po,
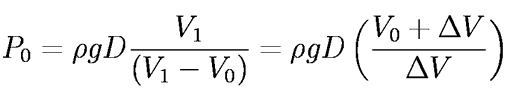
where the final expression uses the measured quantities Vo and the drip volume, 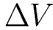
Caveat: When the ball is held in place by atmospheric pressure, the atmospheric pressure on the ball can be greater than the water pressure. The ball's response would be compress until the "spring force" from compression made the net force on the ball equal to zero. This will happen if too much water is allowed to leak out (You can do this by jiggling or spinning the ball), and would give a Po that is less than atmospheric pressure.
-
Contact Information
Contact Number: 717-871-4297
Email: physics@millersville.edu